- Lentivirus
- Adeno-Associated
- Adenovirus
- Pseudovirus
- Vector
- Synthesis
- Autophagy Research
- CRISPR/Cas9
- Noncoding RNA
- Luciferase Assay
- Reagents
WHAT ARE YOU LOOKING FOR?
Lentivirus is a type of retrovirus with a double-stranded RNA genome. Unlike typical retroviruses, lentiviruses have a broader host range and can infect both dividing and non-dividing cells.
Recombinant lentiviral vectors are developed based on HIV-1 (Human Immunodeficiency Virus Type 1) as tool vectors. Their toxic genes have been removed and replaced with exogenous target genes, classifying them as pseudoviruses (with one-time infection capability but no ability to replicate and produce new viruses). These vectors can randomly integrate target genes into the host genome, stably expressing them over several generations in cell lines, allowing for the selection of stably transfected cell lines. Lentivirus packaging service is widely used for gene knockout, gene overexpression, and RNA interference across various cell lines.
Preparation of Reagents and Equipment
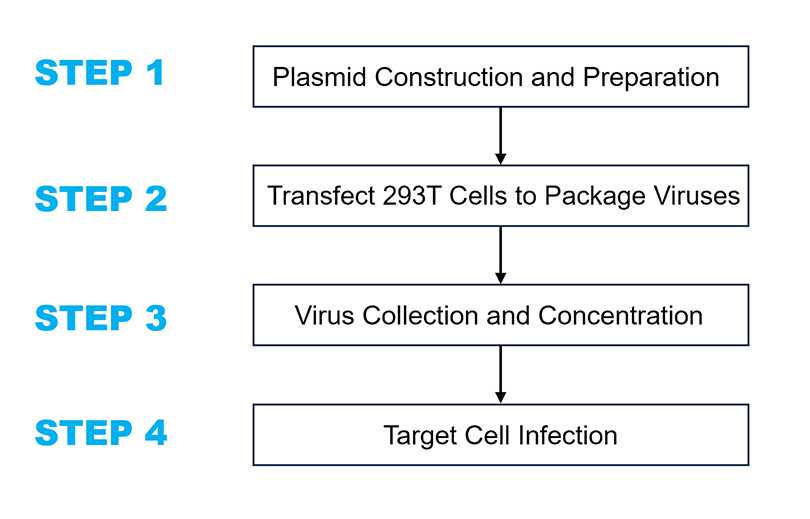
Construct a viral vector containing the target gene and extract high-concentration, high-purity packaging plasmids along with the target plasmid.
Use exponentially growing 293T cells (culture conditions: DMEM + 10% FBS, 37°C, 5% CO₂).
Reagents: fetal bovine serum, DMEM, Opti-MEM, LentiFit, etc.
Instrumentation: fluorescence microscope, biosafety cabinet, ultracentrifuge, etc.
1.Plasmid Amplification: Use a large-scale plasmid extraction kit to de-endotoxin the constructed lentiviral vector and packaging plasmid. The concentration should not be less than 1 μg/μl, with an A260/280 ratio between 1.7-1.8 for virus packaging. A high-quality large-scale extraction kit is recommended, as plasmid quality significantly affects later transfection efficiency and viral titer.
2.Liposome Transfection: The day before transfection, seed healthy 293T cells in a 10 cm dish to achieve a confluence of 30% to 50% the following day. After 24 hours, transfect, ensuring that DMEM and LentiFit™ transfection reagent have returned to room temperature and are mixed well before use. The plasmid composition per dish is as follows:
psPAX2 | 10 μg |
pMD2.G | 10 μg |
pHBLVTM | 10 μg |
The typical molar ratio for the three plasmids used for packaging is 1:1:1, which can be slightly adjusted as needed. Please refer to the LentiFit™ transfection reagent manual for transfection procedures. Replace the medium with fresh culture medium 4-6 hours post-transfection.
3.Virus Collection and Centrifugation: Collect the viral supernatant at 48 hours and 72 hours post-transfection (replace with fresh culture medium after the 48-hour collection). Filter the collected supernatant through a 0.45 μm filter and transfer it to ultracentrifuge tubes, then centrifuge at 72,000 g for 120 minutes at 4°C
4.Virus resuspension and storage. Discard the supernatant and resuspend the viral pellet in 500 μl of resuspension solution. If used within one week, store at 4 ℃; for long-term storage, keep at -80 ℃ or in liquid nitrogen. (Note: The specific resuspension volume should be determined based on experimental needs.)
5.Titration assay. Seed an appropriate number of 293T cells in a 96-well plate. The following day, perform a series of tenfold dilutions of the lentiviral stock and infect the 293T cells. After 24 hours, replace the medium with virus-free culture medium. After 72 hours, observe the results under a fluorescence microscope and count the cells in wells with an appropriate fluorescence ratio (between 10% and 30%).
The titration calculation formula is: Titer (TU/ml) = Cell count × Fluorescence percentage × 10^3 / Volume of viral stock.
293T cells are commonly used as a packaging host, and their health is a key factor affecting virus yield. It is recommended to use 293T cells that are no more than 10 passages in culture. Additionally, 293T cells have poor adherence, so care should be taken when changing the medium to avoid disturbing the cells.
The quality of the plasmid extraction, including concentration and purity, is important. The concentration should not be lower than 1 ng/µL; if the concentration is low, the volume added will increase, which raises the risk of cell contamination.
Lentiviral vectors have a limited capacity for incorporating exogenous genes. Exceeding 3 kb can significantly reduce packaging efficiency; larger plasmids can also lead to decreased transfection efficiency, which in turn affects the titer.
The period from 24 to 48 hours post-transfection is when lentivirus production peaks, allowing for the first harvest of lentivirus. Between 48 to 72 hours post-transfection, the titer declines. It is advisable to collect lentivirus 1 to 2 times using a small volume of culture medium and combine it with the first harvest. If the goal is solely to achieve a high titer, a single harvest is sufficient. In reality, the first harvested lentivirus can generally meet the experimental needs for multiple trials.
Storage of Lentivirus:
(1) Storage conditions: Store in a -80°C freezer. When using the virus, thaw it at 4°C before cell infection.
(2) Avoid repeated freeze-thaw cycles to prevent titer reduction: Each freeze-thaw cycle can decrease the virus titer by approximately 10% to 20%. To avoid this, it is recommended to aliquot the virus based on the amount needed for each use.
(3) If diluting lentivirus titer, take an appropriate amount from the -80°C freezer, thaw it at 4°C, and dilute it to the desired titer using serum-free cell culture medium or 1× PBS (without Ca2+/Mg2+). Diluents should be stored at 4°C and used within three days; aliquoting for storage is not recommended.
As a leading plasmid manufacturing company, Hanbio’s Lentivirus Packaging service exemplifies our commitment to excellence, offering cutting-edge solutions and unparalleled expertise to support your research and therapeutic endeavors. Trust Hanbio to deliver the highest quality viral vectors, tailored to meet your specific needs and drive your scientific breakthroughs.